| Prescrire’s ratings of new products and indications over the past 10 years |
|
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
 |
BRAVO |
| 0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
 |
A REAL ADVANCE
|
| 1 |
0 |
2 |
3 |
1 |
1 |
2
|
1 |
2 |
3 |
 |
OFFERS AN ADVANTAGE
|
| 3 |
6 |
5 |
5 |
5 |
9 |
11 |
10 |
6 |
14 |
 |
POSSIBLY HELPFUL
|
| 14 |
12 |
15 |
15 |
9 |
18 |
22 |
13 |
18 |
19 |
 |
NOTHING NEW
|
| 42 |
48 |
35 |
43 |
56 |
45 |
50 |
61 |
55 |
51 |
 |
JUDGEMENT RESERVED
|
| 7 |
9 |
10 |
6 |
5 |
4 |
5
|
9 |
17 |
12 |
 |
NOT ACCEPTABLE
|
| 15 |
15 |
19 |
15 |
16 |
15 |
9
|
14 |
10 |
9 |
| TOTAL |
82 |
90 |
87 |
87 |
92 |
92 |
99 |
108 |
109 |
108 |
| |
| Therapeutic advances in 2021 compared with the previous 9 years |
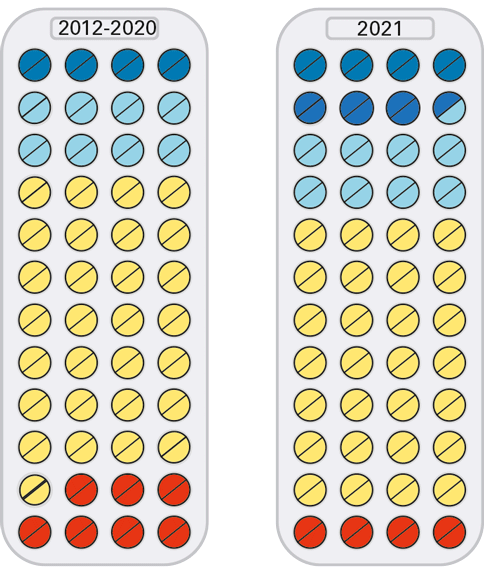 |
| |
 |
Notable advance |
 |
No proven advantages
|
| |
 |
Minimal advance |
 |
More dangerous than useful |
|
|
In summary
Three new drugs, all based on messenger RNA or small interfering RNA technology, represented a major therapeutic advance in 2021.
But the bigger picture is that most of the new authorisations that advanced patient care were adaptations of existing drugs. And that more than half of this year’s new authorisations were not advances, and in fact about one-tenth represented a step backwards compared to existing options.
Every month, Prescrire publishes independent, comparative, systematic reviews of the latest developments in the European pharmaceutical market, including recent marketing authorisations for new active substances, new combinations, new dose strengths, new pharmaceutical forms and new indications.
We also closely monitor news on adverse effects, market withdrawals (instigated by pharmaceutical companies or regulatory authorities), re-introductions of previously withdrawn products, new clinical evaluation data on drugs already on the market, shortages, and the regulatory environment for health products, particularly at EU level.
Our aim is to help subscribers distinguish between genuine pharmaceutical advances and new products or new uses that are no better than existing treatments or should never have been authorised, due to uncertainty over their harms or benefits, or because they are clearly dangerous. |
©Prescrire 1 April 2022
Source: "Prescrire's ratings of new drugs in 2021: a brief review" Prescrire International 2022; 31 (236): 100-102. Free.
|
Enjoy full access to Prescrire International, and support independent information
|
