Prescrire's annual Information Awards are based on the quality of the documentation and information provided by pharmaceutical companies in response to requests by Prescrire's Editorial Staff. We use this documentation when preparing the articles published in the Marketing Authorisations section of our French edition. Prescrire's Information Awards focus on the level of transparence that companies have exhibited over the year in response to our requests for information and documentation.
What information does Prescrire request from pharmaceutical companies?
The information which pharmaceutical companies hold on their drugs, from the earliest stages of development to data collected after their market introduction (or in some cases after their market withdrawal), is important for health care and patient safety. Prescrire primarily asks pharmaceutical companies for: efficacy and safety data; packaging items; and the conditions under which patients can access the drug, in particular whether it is reimbursed (by the French national health insurance system), the planned date of its market introduction or the reasons for its market withdrawal. We compare and contrast the data thus obtained with those gathered from the other sources we consult as part of a systematic search for information, including the documentation provided by health authorities and the scientific literature.
Six companies honoured for their transparency
 |
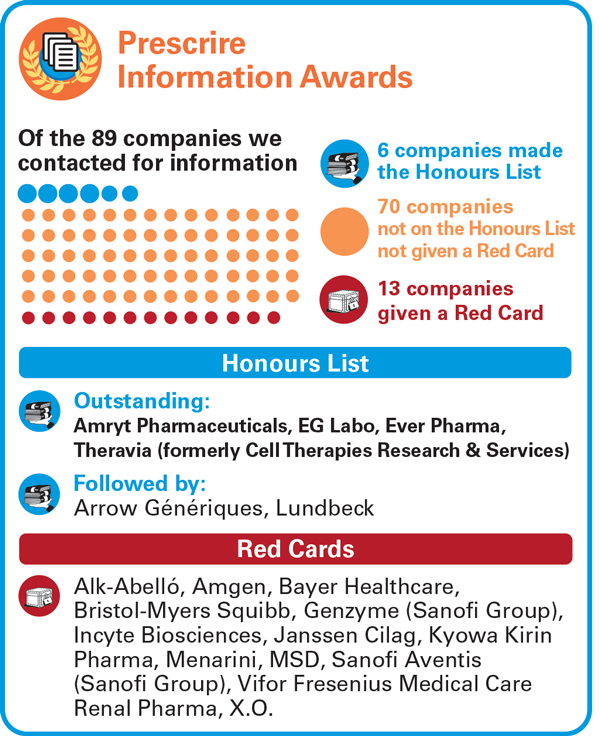
Prescrire requested information from 89 pharmaceutical companies in 2023. Six of them earned a place on the 2023 Information Awards Honours List, by responding with detailed documentation that addressed every aspect of Prescrire’s requests. Four of them were rated as “Outstanding” for sending particularly useful information and documents in a timely manner: Amryt Pharmaceuticals, EG Labo, Ever Pharma, and Theravia (formerly Cell Therapies Research & Services). |
But the vast majority of companies chose secrecy
13 pharmaceutical companies (of the 89 companies we contacted in 2023) chose to withhold information from Prescrire. They failed to respond to repeated requests, some claiming that they do not have time, while others clearly indicated their choice not to provide information to Prescrire. These companies received an information “Red Card”. 70 companies are neither on the Honours List, nor were given a Red Card.
Continue to demand transparency until it becomes a principle
Pharmaceutical companies hold a wealth of documents that they usually make inaccessible to the public, in particular certain evaluation data. European citizens have the right to access clinical data on which marketing authorisations are based. Evaluation data are not confidential data. In fact, the European Ombudsman considered the argument that disclosure of clinical study reports could undermine companies’ commercial interests to be unfounded. In 2023, a minority of pharmaceutical companies showed that a commitment to openness and a company policy of transparency are possible. They serve as examples for all the others to follow.
Download the full article:

FREE "The Prescrire Awards for 2023" Prescrire Int 2024; 33 (259): 132-134. Free.
Back to "The Prescrire Awards for 2023"

|
Enjoy full access to Prescrire International, and support independent information
|
