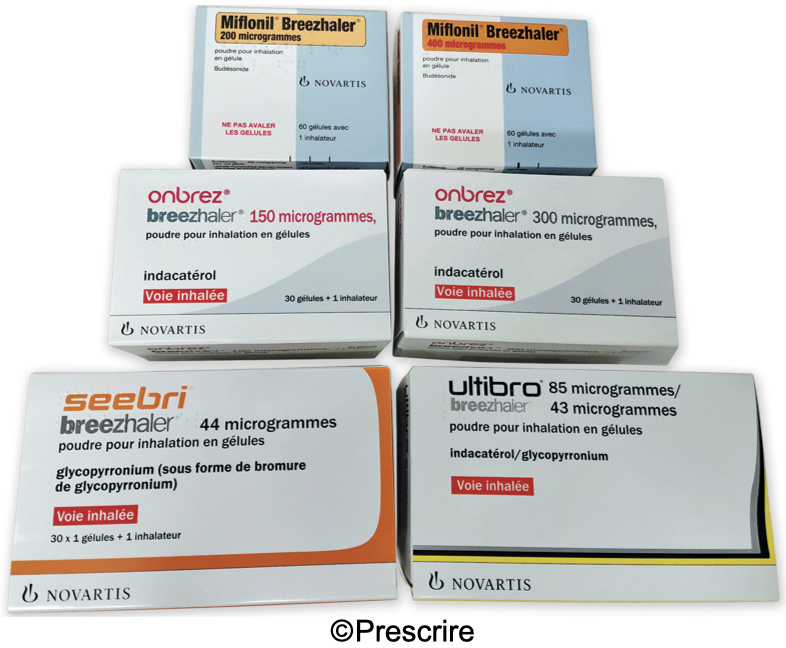
 It is common practice with inhalation therapy in asthma or chronic obstructive pulmonary disease (COPD) for the same inhaler to be supplied with different drugs. For example, in France, since late 2020, six proprietary brands, including four with different compositions, are being marketed with the Breezhaler° device. Nine are being marketed with the Ellipta° inhaler.
It is common practice with inhalation therapy in asthma or chronic obstructive pulmonary disease (COPD) for the same inhaler to be supplied with different drugs. For example, in France, since late 2020, six proprietary brands, including four with different compositions, are being marketed with the Breezhaler° device. Nine are being marketed with the Ellipta° inhaler.
Errors have been reported due to confusion between the brand name of the inhaler and the brand name of the drug. Examples include stating only the name Ellipta° on a prescription and dispensing (after misreading) a different proprietary drug supplied with the same inhaler. Some patients also refer to their treatment by quoting only the name of the inhaler.
The packaging of medicinal products sometimes contributes to the confusion. In France, for example, the name "Breezhaler" appears on the box in letters almost as large as those of the brand name of the drug.
In order to limit such confusion, in addition to stating the dose, all the distinguishing features of these medicines should be written on the prescription, namely: the international non-proprietary name (INN), the proprietary brand name, and, if necessary, the name of the inhaler, for example if drugs are presented under the same brand name but with different inhalers.
At all stages in the treatment process, it is important to check that the drug being dispensed or administered is consistent with all the information on the prescription. It is also important for patients using these treatments to understand the significance of each piece of information, and to remember both names.
©Prescrire 1 November 2021
Source: "Asthma and COPD: risk of confusion between the brand name of the drug and the brand name of the inhaler" Prescrire International 2021; 30 (231): 270. Subscribers only.
|
Enjoy full access to Prescrire International, and support independent information
|
