| Illustrated examples of dangerous drug packaging, from Prescrire's annual packaging review |
| • 20-ml oral syringe: delivers 10 times the paediatric dose |
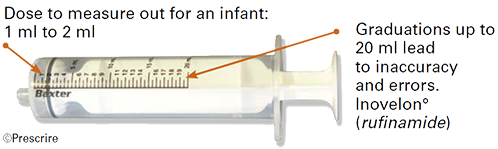
Drugs are often first authorised for use in adults. When their marketing authorisation is later extended to include paediatric use, their packaging is not always adapted accordingly, or is insufficiently adapted. This is a failing of marketing authorisation extension procedures, including European centralised procedures, under the responsibility of the EMA. For example: the capacity of the oral syringe for Inovelon° (rufinamide) was left unchanged, yet it is far too high for infants.
|
• Deroxat° (paroxetine): an inaccurate dosing device (measuring cup) + 2 scales (in mg and ml)
|

Another type of flaw is choosing a graduated measuring cup as a dosing device. Several products examined in 2019 were marketed with a measuring cup, including the antidepressant Deroxat° (paroxetine). The measuring cup for paroxetine had two graduated scales, one in milligrams of the drug and the other in millilitres, a known source of confusion resulting in double or half doses because the drug is a 2 mg/ml suspension.
|
| • Blister packs without, and with, unit-dose labelling |

In France, the ANSM has recommended perforated unit‑dose blister packs as the quality standard for the labelling and safety of all tablets and capsules, whatever drug they contain. But they remained rare among the packaging examined by Prescrire in 2019: for example Baclocur° (baclofen). |
©Prescrire 1 July 2020
Source: "2019 drug packaging review: slow progress and dangers" Prescrire International 2020; 29 (217): 191-195. Free.
|
Enjoy full access to Prescrire International, and support independent information
|
